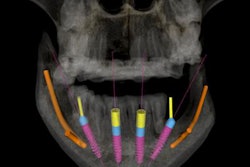
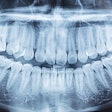
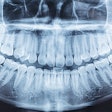
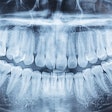
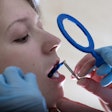
Aspen Imaging has announced U.S. Food and Drug Administration (FDA) approval with dental indications for its AiRTouch portable x-ray system.
According to the manufacturer, the AiRTouch device is lightweight (5.5 lb), and its handheld design and high-performance battery allow for quick and simple flexibility of use in a variety of areas with limited space.
One of the product's unique features, according to the company, is that AiRTouch can acquire images directly to the device, allowing the dentist to review the image first, and then wirelessly transmit images to a review PC via the built-in workstation. Additionally, the device's internal shielding ensures a safe, low dose for both the patient and dentist.