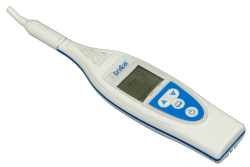
3D Diagnostic Imaging has received 510(k) clearance from the FDA to market the CarieScan Pro in the U.S.
3D Diagnostic Imaging markets the handheld device for the early detection and monitoring of tooth decay device through its subsidiary CarieScan.
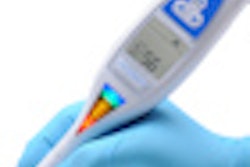
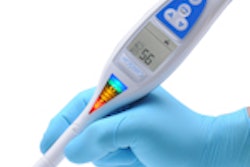
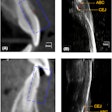
The CarieScan works by electrical impedance. A sensor on the tip of the CarieScan measures the tooth's response to a small alternating current. The results are analyzed by a built-in computer and show up immediately on the unit's pyramid-shaped color LED display, with green indicating sound teeth, yellow indicating initial, and red indicating established caries.
A separate monochrome LCD display provides a numerical readout on the carious condition of the tooth, as well as icons indicating the CarieScan's status (battery charge, wireless connectivity, etc.).
The company claims the system is more than 90% accurate in detecting both sound and carious teeth.