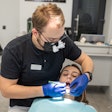
PMI Labs has reduced the price of its OralAdvance early-stage oral cancer detection product by 35% following the completion of its next-generation quantitative cytology analysis platform, ClearCyte.
The redesigned and re-engineered ClearCyte platform allows for faster processing time, increased scalability, and improved overall performance, according to the company. The per-slide scanning time of the machine has been reduced by more than 65%, yielding a yearly scanning capacity of over 40,000 samples per device.
"With the increase of oral cancer cases among young adults, we hope that this initiative will provide more accessibility of OralAdvance to all Canadians, thus allowing health professionals to detect oral cancer in its early stages and provide care for a better chance of survival," said Bohana Turic, president and CEO of PMI, in a press release. "Oral cancer screening should become a standard practice in dental offices nationwide to save lives through early detection."
OralAdvance has received Health Canada approval and the European CE Mark, and is used by dentists to assess the cancerous potential of suspicious lesions in the oral cavity. Cells are collected from areas that look like they may be precancer using the OralAdvance brush. These cells are then assessed at PMI Labs' Vancouver laboratory to determine whether they have abnormal DNA content.
When oral cancer is detected early, the five-year survival rate is 80% to 90% compared to only 50% when detected in its late stage, PMI noted.
Copyright © 2010 DrBicuspid.com