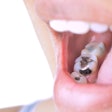
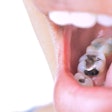
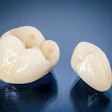
Materials science company Nobio of Israel has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Infinix flowable and bulk-fill composites for restorations.
The composites incorporate the company's patented quaternary ammonium silica-dioxide (QASi) particle technology for maintaining restoration integrity and protecting against bacterial degradation, according to Nobio.
Practitioners can see the composites at the upcoming 2019 ADA annual meeting in San Francisco.